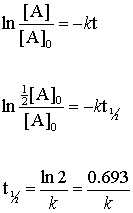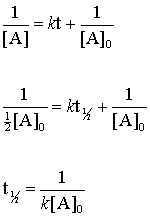half life formula for zero order reaction
The half-life of the reaction is denoted by t 12 and is expressed in seconds. Graphical relations and half lives.

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube
As for other reaction orders an equation for zero-order.

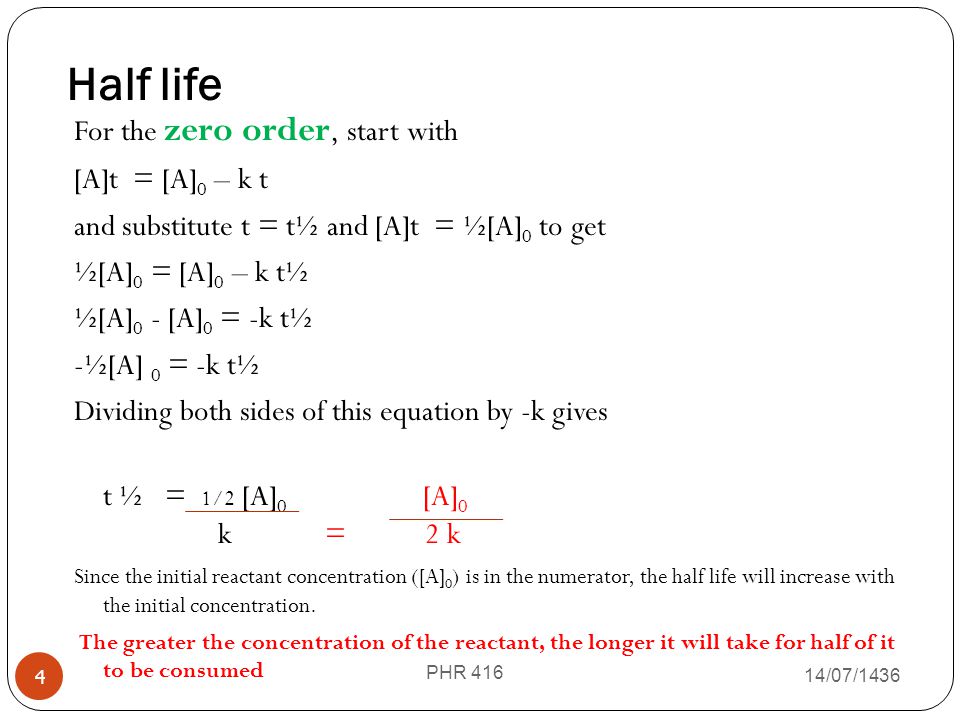
. Where A 0 Initial concentration of reactant at timet 0. The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. Half life means 50 percent of reactants disappear in that time interval.
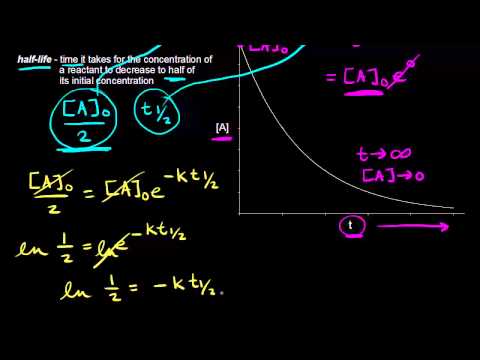
The half-life of a reaction is defined as the time required for the reactant concentration to fall to one half of its initial value. When t t ½ that is the half-life of the reaction completed the concentration of the reactant A A2. The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction.
For a zero order reaction A products rate k. Half life in zero order reaction. Here is how the Half Life of Zero Order Reaction calculation can be explained with given input values - 1 2000 21000.
Converting a half life to a rate constant. For the first-order reaction the half-life is defined as t 12 0693k. NA Product The rate law of zero order kinetics is.
For a zero-order reaction the integrated rate law is. Remember the half-life of a reaction changes with the order of the reaction. Half-Life of a Zero Order Reaction.
It is represented by t12. The half-life for zero-order and second-order reactions half-life changes based on the concentration of the reactant. In a second-order reaction the half-life of the reaction is inversely proportional to the.
Half-life is denoted by the symbol t 12. Rate k C12H22O11 Half-Life of a reaction t12. Equations for Half Lives.
The integrated rate constant for the zero-order reaction is given by. Determining a half life. It is the time in which the concentration of a reactant is reduced to one half of its initial concentration.
1A n-1 1 A 0 n-1 n-1 kt. This is an expression of the half-life of a zero-order reaction. And for the second-order reaction the formula for the.
The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction is given as t12 0693k The half-life of a second-order reaction is given by the formula 1kR0. Given below is the half-life of a zero-order reaction. Unlike a first-order reaction in a zero- or second-order reaction the half-life is dependent on the initial concentration ie.
T ½ 1 k A o Top. The rate constant k will have units of concentrationtime such as Ms due to. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant.
T ½ A o 2k For a first order reaction A products rate kA. Not a set value that we can calculate. Thus for t t 12 A t ½ A o.
Replace t with half-life t 12. Half-Life of Zero Order Reaction. Our video lecture will discuss the Integrated rate equation for Zero Order reaction from Chemical Kinetics for upcoming Board Exams NEET IIT-JEE Preparati.
From the above formula the half-life of the zero order kinetics depends on the initial concentration of the reactant. The timescale in which there is a 50 reduction in the initial population is referred to as half-life. A A 0 - kt.
By definition the half-life of any of the reactions is the amount of time the reactants take to consume half of the starting material. The half-life of a first-order reaction is given as t 12 0693k. Thus for a first-order reaction each successive half-life is the same length of time as shown in.
The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k. It is to be noted that the half-life of a zero-order reaction is determined by the initial concentration and rate constant. 5 rows Zero-Order Reactions.
T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. The rate constant for a. For a general reaction.
The half-life of a zero-order reaction the formula is given as t 12 R 02 k The half-life of a first-order reaction is given as t 12 0693k The half-life of a second-order reaction is given by the formula 1kR 0. The rate constant for a zero-order reaction is measured in molL -1 s -1. Therefore A2 k 0 t ½ or t ½ A2k.
The half-life of a second-order reaction is given by the formula 1kR 0. Where The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R 0 in molL-1 or M is R 0 and. As for other reaction orders an equation for zero-order.
The rate constant for a Zero-order reaction rate of constant k. Now we have the following equation and can solve for eqt_ 12 eq. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.
Half life in zero order reaction. The rate constant for a zero-order reaction is 054 M-1s-1. To use this online calculator for Half Life of Zero Order Reaction enter Initial concentration for zero order reaction C0 Rate constant of zero order reaction k and hit the calculate button.
Half life formula for nth order reaction. Half-Life of a Zero Order Reaction. T12 A 02K.
Frac 1 A_02 frac 1 A_0 kt_ 12 frac 1 A_02 - frac 1 A_0 kt_ 12. From the integral form we have the following equation. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2.
½ A A 0 kt 12. And we typically use the concept of half-life to for example determine the age of ancient artifacts or predict when a radioactive sample will be safe to handle.

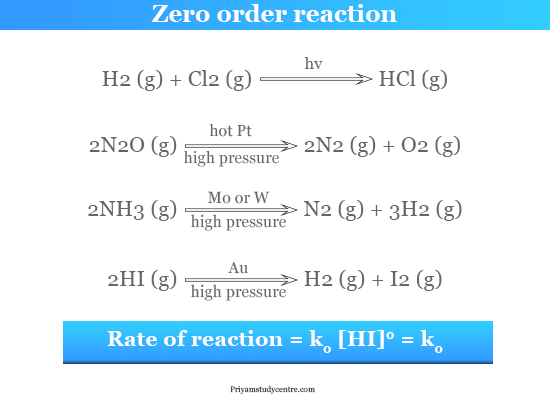
Zero Order Reaction Definition Examples Formula

Half Life Of Zero Th 0th Order Reaction Derivation Youtube

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com
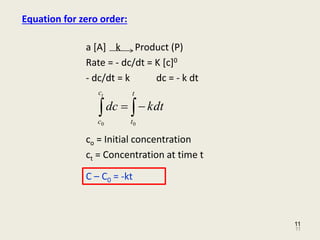
Kinetics And Drug Stability Ed

Half Life Expressions Chemistnate

Zero Order Reactions Video Kinetics Khan Academy

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com

Integrated Rate Laws Chemistry For Majors

Which Of The Following Statements Are Corrects

Half Life Expressions Chemistnate
Principles And Kinetics Of Drug Stability Phr 416 Ppt Video Online Download

Half Life Expressions Chemistnate
Half Life Of A First Order Reaction Video Khan Academy